Small molecules dominate approvals. Explore how pharma contract manufacturing companies enable scale and compliance.
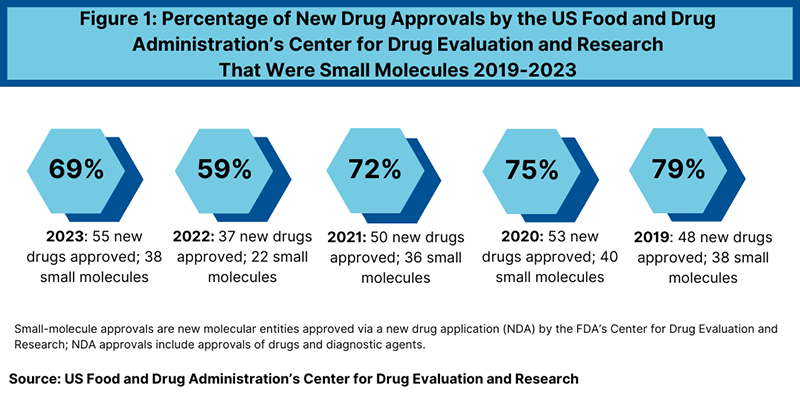
In 2023, the US Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) witnessed a notable surge in new drug approvals compared to the previous year. With 55 new molecular entities (NMEs) and new therapeutic biologics approved, representing a 49% increase over 2022, the pharmaceutical landscape experienced a significant uptick in innovation. Notably, small-molecule drugs continued to dominate these approvals, raising questions about their market performance and translating potential.
Small-Molecule New Drug Approvals
Of the total new drug approvals in 2023, small-molecule products accounted for 69%, signaling a consistent trend in recent years. Despite a dip in 2022, where small-molecule approvals represented only 59% of the total, the numbers rebounded, aligning with historical averages between 2018 and 2021. However, the increase in small-molecule approvals in 2023 mirrors a broader shift, especially following the decline observed in the previous year.
Small-Molecules and First-in-Class Drugs
Beyond sheer numbers, the innovation landscape is also evaluated based on “first-in-class” drugs—those with novel mechanisms of action compared to existing treatments. In 2023, CDER approved 20 first-in-class drugs, constituting approximately 36% of total approvals. Strikingly, 85% of these first-in-class drugs were small molecules, indicating their continued significance in pioneering therapeutic advancements.
Insights into First-in-Class Small-Molecule Drugs
A deeper dive into first-in-class small-molecule drugs reveals a nuanced picture. While they dominate the innovation frontier, a considerable portion targets niche indications, particularly orphan or rare diseases affecting fewer than 200,000 individuals in the US. This trend underscores the pivotal role of small molecules in addressing unmet medical needs and driving advancements in specialized therapeutic areas.
Case Studies: Notable First-in-Class Small-Molecule Drugs
Several large to mid-sized bio/pharma companies spearheaded the development of first-in-class small-molecule drugs in 2023. Notable examples include Astellas’ Veozah for menopausal symptoms, AstraZeneca’s Truqap for advanced breast cancer, and Pfizer’s Paxlovid for COVID-19 treatment. These innovations highlight the diverse therapeutic areas and the industry’s commitment to addressing complex health challenges.
Balancing Innovation and Market Viability
While small-molecule drugs continue to lead in innovation, their market success remains a subject of scrutiny. As they navigate the regulatory landscape and address niche indications, the industry must strike a delicate balance between innovation and commercial viability. Ultimately, the continued evolution of small-molecule drugs underscores their resilience and potential to shape the future of pharmaceutical therapeutics.
Source: Manufacturing & Supply Lines: Small-Molecule APIs
Pharma Contract Manufacturing Companies
Pharma Contract Manufacturing Companies
Comments are closed.