Learn how CDMO services are fueling growth in the contract ADC market with innovation and global capacity.
The contract market for antibody drug conjugates (ADCs) is set for double-digit growth, driven by significant investments from both large bio/pharma companies and smaller ADC innovators. This surge in demand for high-potency manufacturing and conjugation services raises the question: which companies are leading the charge?
Contract ADC Market Expansion
Investment in high-potency manufacturing remains a key focus for CDMOs/CMOs. This investment is largely fueled by the robust growth in the ADC market as both large and small bio/pharma companies advance their pipelines, particularly in oncology, which is the primary therapeutic area driving this expansion.
Recent high-profile acquisitions highlight the intense interest in ADCs. Notable deals include Pfizer’s $43 billion acquisition of Seagen, a bio/pharma company based in Bothwell, Washington, with several approved ADCs and key pipeline candidates. AbbVie’s $10 billion acquisition of ImmunoGen in February 2024 and Johnson & Johnson’s $2 billion acquisition of Ambrx Biopharma in March 2024 further emphasize this trend. Significant drug-development partnerships include Merck & Co. and Daiichi Sankyo’s $22 billion global licensing and commercialization agreement for three ADC candidates announced in October 2023, and Daiichi Sankyo’s $6 billion ADC partnership with AstraZeneca from 2020.
ADCs combine biologic and small-molecule components, typically involving a cytotoxic small-molecule compound linked to a monoclonal antibody for oncology applications. CDMOs/CMOs specializing in high-potency manufacturing require specialized high-containment conditions for producing the cytotoxic compounds and conjugation capabilities to link these compounds to monoclonal antibodies.
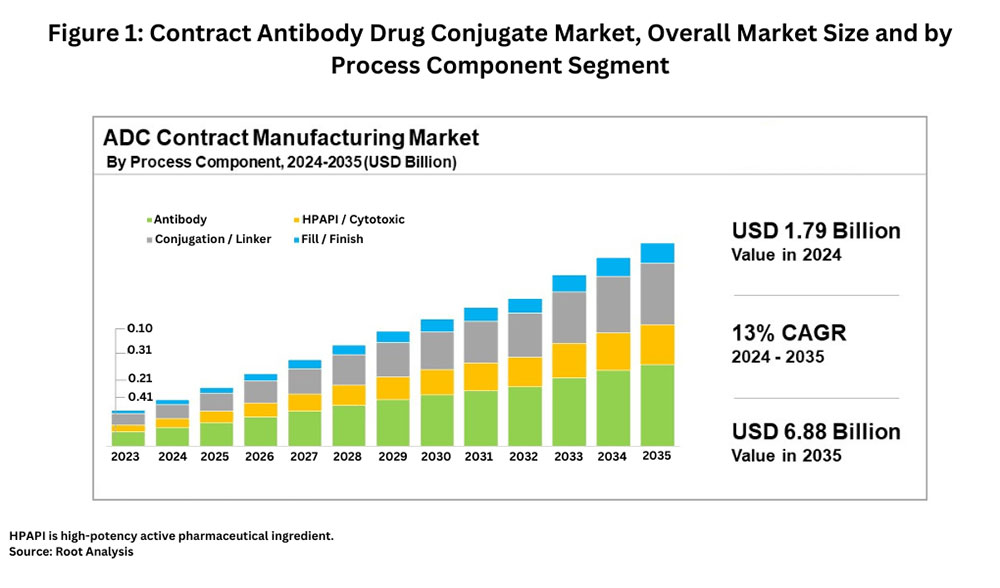
According to a recent market study by Root Analysis, the global ADC contract manufacturing market, valued at $1.79 billion in 2024, is projected to grow at a compound annual growth rate of 13%, reaching approximately $6.87 billion by 2035. The contract ADC market is segmented into four major components: the antibody, high-potency active pharmaceutical ingredient (HPAPI)/cytotoxic payload, conjugation/linker, and fill–finish of the final drug product. The antibody segment holds the largest market share at approximately 40%, followed by the conjugation/linker segment at 30%, the HPAPI/cytotoxic payload segment at 20%, and the fill–finish segment at 10%, according to the Root Analysis study.
Figure 1 details the market size, growth rates, and segments for the contract ADC market.
Surge in Bio/Pharmaceutical M&A for ADCs
Pfizer’s $43-billion acquisition of Seagen in December 2023 stands out not only as the largest ADC deal but also as the biggest industry acquisition of the year. Seagen’s portfolio boasts four approved medicines targeting solid tumors and hematologic malignancies, including three ADCs: Adcetris (brentuximab vedotin), Padcev (enfortumab vedotin), and Tivdak (tisotumab vedotin). Additionally, Seagen commercializes Tukysa (tucatinib), approved in 2023 for certain types of colorectal cancer in combination with trastuzumab. Ongoing clinical development programs for these medicines aim to explore new tumor types and expanded indications, with significant milestones expected annually through 2027.
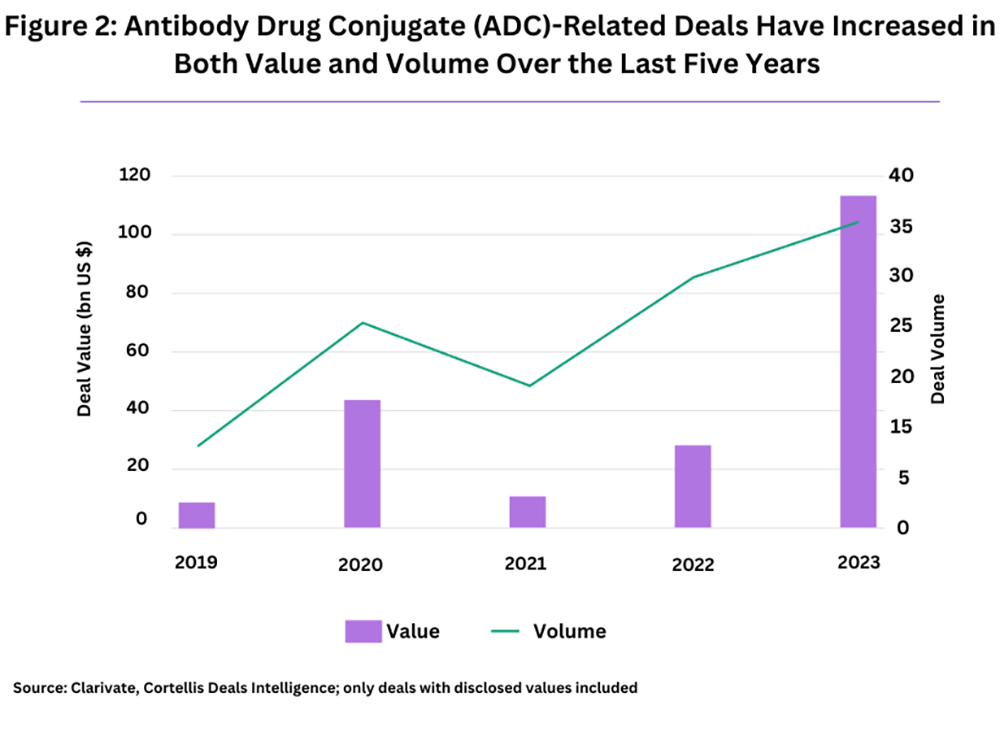
Over the past five years (2019-2023), there have been 15 ADC-related mergers and acquisitions (M&A), as highlighted by a recent analysis from Clarivate, a business intelligence firm. Nearly half of these, seven in total, occurred in 2023 alone.
So far in 2024 (as of mid-April), notable ADC-related M&A deals include AbbVie’s $10-billion acquisition of ImmunoGen. This deal brought AbbVie Elahere (mirvetuximab soravtansine-gynx), a commercial ADC for platinum-resistant ovarian cancer. ImmunoGen’s pipeline also features IMGN-151, an anti-FRα ADC in Phase I for ovarian cancer, with potential applications in other solid tumors. Pivekimab sunirine, an anti-CD123 ADC targeting blastic plasmacytoid dendritic cell neoplasm (BPDCN), is in Phase II and has received FDA breakthrough therapy designation for treating relapsed/refractory BPDCN.
Additionally, Johnson & Johnson completed a $2-billion acquisition of Ambrx Biopharma, acquiring several clinical and preclinical ADC programs, including ARX517 for prostate cancer, ARX788 for breast cancer, and ARX305 for renal-cell carcinoma.
Another significant ADC-related M&A was Genmab’s $1.8-billion acquisition of ProfoundBio (Suzhou) Co. Ltd., a Copenhagen-based bio/pharmaceutical company.
Rising ADC-Related Deal-Making
Beyond acquisitions, the ADC field has seen an increase in high-value partnering deals among bio/pharma companies. The Clarivate analysis notes 33 disclosed blockbuster deals (valued at $1 billion or more) in the past five years (2019-2023), signaling a substantial rise in activity and valuation in the ADC sector (see Figure 2).
Oncology Dominates ADC-Related Deal-Making Activity
Over the past decade (2014-2023), oncology has led the way in ADC-related deals, both in terms of volume and value, accounting for 392 deals with a total projected valuation of $206 billion, according to a Clarivate analysis. In comparison, the next largest therapeutic sector in ADC-related deals is infectious diseases, with nine deals valued at approximately $806 million.
One significant transaction was the $22-billion global development and commercialization agreement between Merck & Co. and Daiichi Sankyo, announced in October 2023. This agreement covers three of Daiichi Sankyo’s ADC oncology drug candidates: patritumab deruxtecan, ifinatamab deruxtecan, and raludotatug deruxtecan. Merck committed to a $4-billion upfront payment, $1.5 billion in continuation payments over the next 24 months, and potential additional payments up to $16.5 billion contingent on achieving future sales milestones, totaling up to $22 billion. These programs have the potential for multi-billion dollar worldwide commercial revenue by the mid-2030s. The companies will jointly develop and potentially commercialize these ADC candidates globally, except in Japan, where Daiichi Sankyo retains exclusive rights and will be solely responsible for manufacturing and supply.
However, the companies faced a setback with patritumab deruxtecan when the FDA issued a Complete Response Letter (CRL) in June 2024 due to issues found during an inspection of a third-party manufacturing facility. The CRL did not raise concerns about the drug’s efficacy or safety data. The companies plan to work with the FDA and the third-party manufacturer to resolve these issues.
Merck’s partnership with Daiichi Sankyo follows its $9.5-billion license and collaboration agreement with Kelun-Biotech, a Chengdu, China-based bio/pharma company, established in 2022 to develop seven preclinical ADCs for cancer treatment. This deal was the largest in terms of value in 2022 and second over the past five years (2019-2023), according to Clarivate. All top ADC deals in this period focused on oncology.
Daiichi Sankyo is also partnered with AstraZeneca in a $6-billion ADC deal from 2020 to develop and commercialize datopotamab deruxtecan, targeting multiple tumor types, including breast and lung cancer. The drug is under regulatory review in the US for a specific form of breast cancer, with an expected review date of January 29, 2025. In March 2024, the European Medicines Agency (EMA) validated two marketing authorization applications for the drug to treat certain forms of breast and lung cancer, initiating the scientific review process. Clarivate projects this ADC as a potential blockbuster with expected combined sales of $2.70 billion for breast cancer and non-small-cell lung cancer by 2029, as per Clarivate’s Drugs to Watch 2024 report.
In 2024 alone (as of April 12), 32 ADC-related deals have been announced or completed, according to Clarivate. The largest disclosed deals (over $500 million) include:
- Bristol-Myers Squibb and Systimmune, for a projected $8.4 billion to co-develop and co-commercialize Systimmune’s BL-B01D1 in the US for treating metastatic or unresectable non-small-cell lung cancer.
- Merck KGaA and Caris Discovery, for a projected $1.4 billion to identify targets and develop ADCs for cancer worldwide.
- Roche and MediLink Therapeutics, for a projected $1 billion to develop, manufacture, and commercialize MediLink Therapeutics’ YL-211 against solid tumors worldwide.
- Ispen and Sutro Biopharma, for a projected $899 million to develop and commercialize Sutro Biopharma’s STRO-003 for treating solid tumors.
ADC Market: Clinical Development
Clarivate estimates that over 1,200 active clinical trials for ADCs are ongoing, with most initiated in the past five years. Although cancer remains the primary focus, 13 non-oncology ADC assets are being evaluated in clinical trials started within the last five years, covering indications such as age-related macular degeneration (AMD), AL amyloidosis, Crohn’s disease, diabetic macular edema, Duchenne muscular dystrophy, influenza, kidney disease, rheumatoid arthritis, nonalcoholic steatohepatitis (NASH), scleroderma, familial amyotrophic lateral sclerosis, and hepatitis B virus infection. Among these, Kodiak Sciences’ KSI-501 for AMD is the furthest along, with a Phase III trial slated to begin in June 2024.
Emerging ADC Companies to Watch
Clarivate highlights seven lesser-known companies specializing in ADCs, showing promise through their pipeline assets or ADC-related technologies:
- Adcendo ApS (Frederiksberg, Denmark) develops ADCs targeting the uPARAP receptor in various cancers, aiming to replace conventional chemotherapy with targeted treatments.
- Araris Biotech AG (Zurich, Switzerland) offers proprietary conjugation technology and linker-payload platforms for creating efficacious and less toxic ADC cancer treatments.
- GO Therapeutics (Natick, Massachusetts) uses advances in glycoproteomics to develop antibody-based therapies against cancer.
- Heidelberg Pharma AG (Munich, Germany) utilizes amanitin-based compounds as ADC payloads, targeting RNA polymerase II to induce apoptosis.
- Pheon Therapeutics (Cambridge, Massachusetts) focuses on novel oncology targets and proprietary linker-payload technologies, with its lead program PHN-010 entering clinical stage in 2024.
- Tallac Therapeutics (Burlingame, California) uses novel oligonucleotide payloads to create antibody-oligonucleotide conjugates (AOCs) for targeted immune activation within tumors.
- Tubulis (Planegg, Germany) employs proprietary Tub-tag and P5 conjugation platforms to deliver targeted ADCs, minimizing offsite toxicities.
The surge in ADC-related M&A and partnering deals, particularly in oncology, showcases the growing significance and potential of antibody-drug conjugates in the bio/pharmaceutical industry. Major acquisitions, such as Pfizer’s purchase of Seagen and strategic collaborations like Merck & Co.’s agreement with Daiichi Sankyo, highlight the intense focus and substantial investment in this area. With over 1,200 active clinical trials and an increasing number of blockbuster deals, the ADC market is poised for robust growth. As emerging companies continue to innovate and develop novel ADC technologies, the landscape of cancer treatment and other therapeutic areas will likely be transformed, offering more targeted, efficacious, and less toxic treatment options for patients worldwide. The momentum in ADC-related activities reflects a promising future, driven by ongoing advancements and strategic investments in this cutting-edge field.
CDMO Services
CDMO Services
Comments are closed.












