Learn about EU drug supply chain risks and how pharma supplier directories support onshoring and resilience.
In response to growing concerns over medicine shortages and supply chain vulnerabilities, the European Union is taking proactive measures to safeguard its pharmaceutical sector. The European Commission, along with key agencies like the Heads of Medicines Agencies (HMA) and the European Medicines Agency (EMA), has initiated a comprehensive evaluation of the EU drug supply chains. This initiative aims to address the root causes of shortages, enhance the security of medicine supplies, and explore the potential for bolstering domestic manufacturing capabilities. As part of this effort, a pilot study was conducted to assess the vulnerabilities of critical medicines and provide strategic recommendations for improving the resilience of the EU’s pharmaceutical supply network. This article delves into the findings of this study and examines the implications for the future of drug supply and manufacturing in the EU.
Evaluating Drug Supply Chains in the EU
The European Commission is taking decisive steps to address medicine shortages within the European Union (EU). This initiative includes a comprehensive set of short-term and long-term actions aimed at enhancing the security of medicine supply across the EU. The primary objective is to coordinate efforts with all relevant stakeholders to prevent or mitigate shortages, particularly focusing on the most critical medicines that require assured security of supply. This initiative builds on ongoing efforts, including the proposed revision of the EU pharmaceutical legislation, marking the first major reform in over two decades.
A crucial aspect of this evaluation is understanding the vulnerabilities within the drug supply chain and the manufacturing and supply of active pharmaceutical ingredients (APIs) and related inputs, both within and outside the EU. The European Commission has highlighted the need to bolster the resilience of these supply chains, emphasizing the importance of securing key capabilities at various levels—from sourcing raw materials and API production to the manufacturing of finished products. Additionally, there is a call to reassess manufacturing dependencies and strengthen the EU’s strategic autonomy in health by exploring potential expansions in internal manufacturing capacity and enhancing strategic partnerships with neighboring and like-minded countries globally.
Pilot Study on EU Drug Supply Chains
In December 2023, the European Commission, in collaboration with the Heads of Medicines Agencies (HMA) and the European Medicines Agency (EMA), released the first version of the Union list of critical medicines. This list comprises over 200 active substances deemed critical based on therapeutic indications and the availability of suitable alternatives within the EU/European Economic Area (EEA). Following the publication of this list, the European Commission conducted a pilot study to assess the supply chain vulnerabilities of a select group of 11 critical medicines from the list. The goal was to guide coordinated actions at the EU level.
The medicines chosen for the pilot study included alteplase, amoxicillin, amoxicillin/clavulanic acid, benzathine benzylpenicillin, clonazepam, fludarabine, glucagon, hepatitis B vaccine, rifampicin, verteporfin, and vincristine. Data was collected from marketing authorization holders, EU member states, and the EMA regarding the supply chains of these medicines. In June 2024, the European Commission published the results of this pilot study, marking a crucial step in evaluating the vulnerabilities of critical medicine supply chains. The findings aim to inform discussions among stakeholders and EU decision-makers on how to address medicine shortages and potentially reduce reliance on non-EU-based drug supplies. The study also provides recommendations for addressing shortages from an industrial policy perspective.
Information and Data Evaluated in the Pilot Study
The pilot study utilized a data-driven approach to identify a subset of critical medicines from the HMA/EMA Union list. The selection process balanced public health needs with potential supply chain vulnerabilities. A workshop in January 2024, involving EU member states, industry associations, the EMA, and other Commission services, helped establish the criteria for selecting the initial tranche for the vulnerability assessment. Historical shortages emerged as a key criterion, given its relevance and data availability, alongside other factors like API producer locations and market concentration.
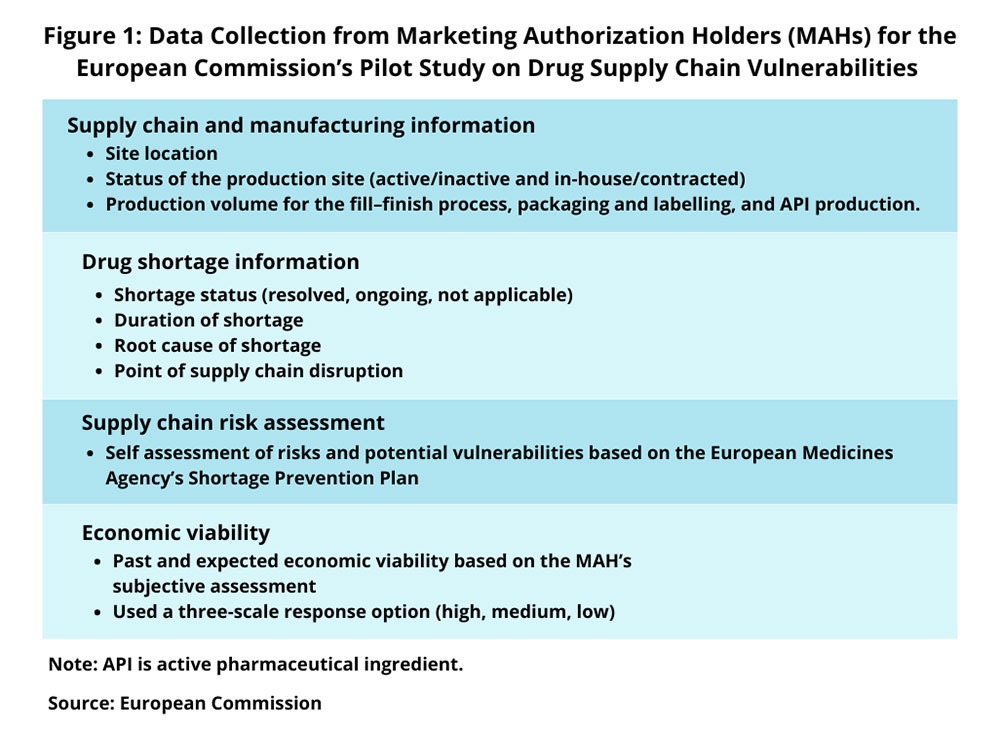

The study involved identifying key suppliers based on 2022 sales data across the EU/EEA. Suppliers were ranked by market share, ensuring broad geographical coverage. A total of 49 key suppliers for the 11 selected medicines were identified. Information was collected through questionnaires sent to marketing authorization holders and EU member states, and supplemented by data from the EMA on shortages and manufacturing sites. This information was refined based on feedback from workshops and online meetings with stakeholders.
The data collected included details on supply chain and manufacturing, production site status, and historical shortage information. Key indicators such as EU industrial presence, manufacturer and site diversification, supply chain risks, market concentration, unpredictable demand, and economic viability were analyzed to assess risk levels.
Results of the Pilot Study
The pilot study revealed significant insights into the supply chain vulnerabilities of the 11 medicines. Key findings include:
- 1. EU Industrial Presence: Four out of the 11 substances showed high vulnerability due to a significant dependence on API production and supply sources outside the EU. This highlights a critical risk related to non-EU dependencies.
- 2. Supply Chain Risks: For three of the medicines, marketing authorization holders reported high risks, linked to low diversification and reliance on a limited number of suppliers. The remaining eight substances were associated with medium risks in their supply chains.
- 3. Economic Viability: Four substances faced significant economic challenges, including cost pressures, high competition, energy costs, inflation, and unsustainability of the market due to prices being below costs in certain EU member states.
The study also highlighted that shortages were primarily due to manufacturing issues and unexpected increases in demand. It noted that many shortage notifications lacked specificity regarding root causes. Additionally, all 11 medicines had over 30% of their supply from a single country or manufacturer, which increases vulnerability to geopolitical issues, trade restrictions, or production problems.
The variable resilience of manufacturing sites among the substances suggests a need for targeted strategies to enhance production site diversification. The study also indicated that unpredictable demand poses challenges for supply chain planning, necessitating more flexible and responsive systems. Addressing economic viability challenges is crucial for maintaining the health and sustainability of these supply chains.
Conclusion
The pilot study conducted by the European Commission, HMA, and EMA marks a significant step in understanding and addressing the vulnerabilities within the EU drug supply chain. By identifying critical areas of concern and providing strategic recommendations, the study aims to enhance the resilience of the EU’s medicine supply and reduce dependence on non-EU sources. The results underscore the need for continued efforts to strengthen internal manufacturing capabilities and explore strategic partnerships to ensure the security of medicine supply in the EU.
Pharma Supplier Directory
Pharma Supplier Directory
Comments are closed.











