Discover how pharmaceutical contract manufacturing helps accelerate cancer treatment delivery worldwide.
Big pharmaceutical companies that manufacture personalized blood cancer treatments are working diligently to cut the manufacturing turnaround time by as much as half in the coming years, aiming to deliver these therapies sooner in a patient’s disease course.
These treatments, known as CAR-T therapies, are specifically designed for the sickest patients for whom standard treatments have failed. The process involves removing a patient’s T cells – a critical component of the immune system – and re-engineering them to recognize and attack malignant cells once they are re-infused into the patient.
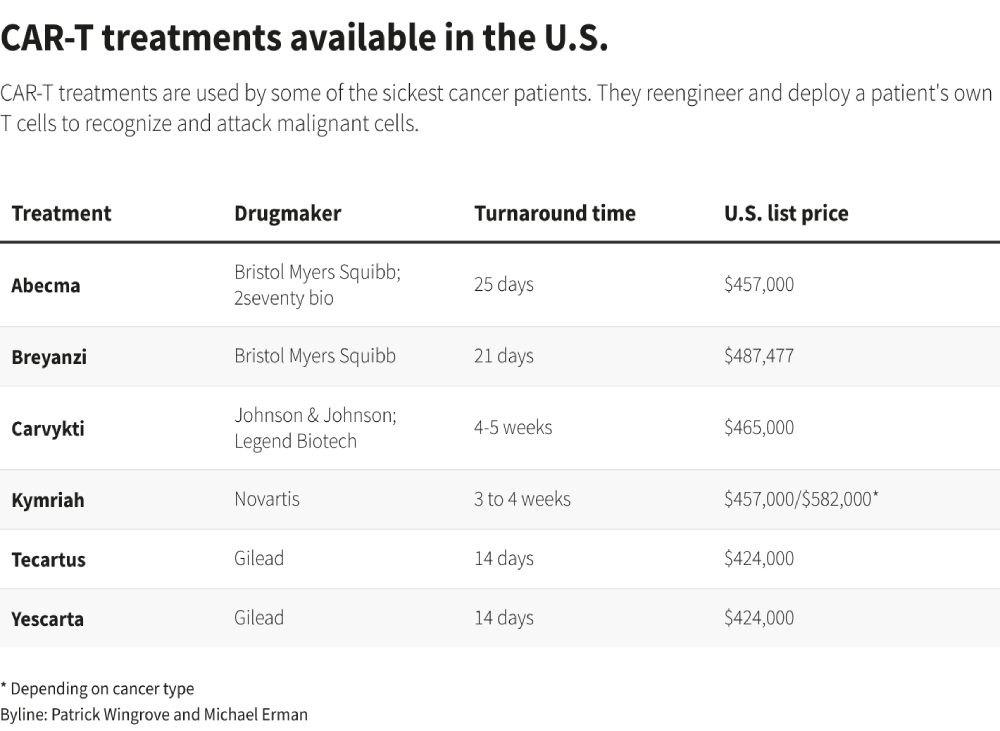
Leading pharmaceutical companies like Gilead Sciences, Novartis, Johnson & Johnson, and Bristol Myers Squibb have made billions from their CAR-T therapies. These treatments can extend the lives of patients with aggressive, advanced cancers by months or even years.
Reducing Turnaround Time: A Critical Need
Trimming days or even weeks from the time it currently takes between removing and reintroducing the cells to a patient could significantly benefit those who might otherwise become too sick during the process, according to doctors, the companies, and analysts.
Prices for the one-time treatment range from $400,000 to nearly $600,000, with profit margins estimated at about 50%. Therefore, expanding the patient population that can receive these therapies is not insignificant. According to Oppenheimer & Co analyst Hartaj Singh, only around 1 in 5 eligible patients currently receive CAR-T treatments.
“It’s complex manufacturing using the patient’s own cells, but because some of the responses reported are just so long and durable, physicians will go to it,” Singh said. “It’s the biggest gun out there.”
When the first CAR-T therapy was launched by Novartis in 2017, the time to treatment was as long as 37 days. Today, Gilead has managed to reduce this to 14 days. Further improvements in automation and regulatory adjustments could help companies trim this time even more.
Singh mentioned that physicians suggested to him that a one-week turnaround could expand these treatments to 2 or 3 out of 5 eligible patients. Improvements in manufacturing time could provide a competitive edge when therapies are approved for the same cancer or population and directly compete. It could also give companies an advantage over more quickly available treatments such as antibodies or antibody-drug conjugates.
Innovations and Future Plans
To create these treatments, a patient’s T cells are isolated, frozen, and shipped to manufacturers. Once received, the cells are purified, modified, and expanded to a large enough number to treat the patient. They then go through quality control processes before being re-frozen and shipped back for infusion.
Gilead is testing new technology that it hopes can cut two days from the manufacturing process by using healthier and more potent younger cells. The company is also looking to fully automate some parts of manufacturing that are currently semi-automated and gain regulatory approval to shorten the quality control time.
Novartis aims for 10 days or less in the U.S. for its next generation of treatments through its rapid manufacturing platform T-Charge. Its treatment, Kymriah, currently takes 3 to 4 weeks with a target of 22 days. The T-Charge system would shorten the manufacturing time because it allows for the engineered cells to multiply within the patient, reducing the need for extended cell expansion before infusion.
Every Day Matters
Dr. Chijioke Nze of MD Anderson Cancer Center in Houston emphasized that a manufacturing time of a week would be ideal. Frailer patients can develop kidney or liver dysfunction while they wait for their therapy to be manufactured or may become too weak to successfully undergo treatment. He has prescribed Gilead’s Yescarta and Tecartus as well as Bristol Myers’ Breyanzi.
“The patient population that needs CAR-T cell therapies generally have the more aggressive of an already aggressive disease,” Nze said.
Analysts and drugmakers agree that using CAR-T drugs earlier, before doctors prescribe multiple other treatments that fail, would also expand their use. For example, J&J’s Carvykti was approved in April for patients who did not benefit from just one other treatment for multiple myeloma. Some CAR-T approvals require patients to receive three or four other treatments to fail before using the therapy.
Increasing the number of hospitals and health centers offering the treatments will also make it feasible for more patients. Since 2017, over 42,000 people globally have received CAR-T treatments. Six are approved in the U.S. for the treatment of blood cancers, including lymphomas and some forms of leukemia.
Expanding Capacity and Reducing Treatment Times
Lynelle Hoch, who leads Bristol Myers Squibb’s cell therapy business, said the company’s current focus is on increasing manufacturing capacity. She noted that the time to produce the treatments is trending lower and will be further helped by its collaboration with Cellares, a development and manufacturing organization with a fully automated cell therapy production platform.
J&J has doubled its CAR-T manufacturing capacity last year and is striving to double it again in 2024. It is working to further reduce treatment times. Cindy Perettie, an executive at Gilead’s Kite cell therapy unit, stated that lymphoma patients who have already tried two treatments generally only have months to survive and need to get treated as quickly as possible.
“Every day matters for these patients,” Perettie emphasized.
As pharmaceutical companies continue to innovate and streamline the manufacturing process, the hope is to make these life-saving therapies more accessible and effective for patients battling aggressive cancers.
Pharmaceutical Contract Manufacturing
Pharmaceutical Contract Manufacturing
Comments are closed.











